The Restoration of Vitamin C Synthesis in Humans

Vitamin C
by Thomas E. Levy, MD, JD and Ron Hunninghake, MD
OMNS (May 11, 2022) The full importance of vitamin C in reaching and maintaining optimal health remains largely unappreciated by most traditional health care practitioners. Certainly, it is widely appreciated that vitamin C is required to sustain life, and that its severe deficiency in the body reliably results in the development of scurvy, with death following shortly thereafter in the absence of new vitamin C intake. However, it is not widely appreciated that optimal human metabolism requires the assimilation of new multigram (macronutrient) quantities of vitamin C on a daily basis, not just 50 to 100 milligram (micronutrient) quantities. Ironically, much of the reason for this misconception comes from vitamin C (ascorbic acid) being regarded solely as a “vitamin” in the first place, when it should be primarily regarded as the singularly most important nutrient in the body.
A vitamin is defined as an organic molecule needed in limited quantities for the proper functioning of the organism. Furthermore, a vitamin is considered to be an essential micronutrient available only through diet, unable to be synthesized in the organism in sufficient amounts. As a result, it is much better to regard vitamin C as follows:
Vitamin C is the primary macronutrient in the body, although it also possesses vitamin-like qualities, as very small amounts are needed to avoid the development of the associated deficiency disease (scurvy). And in the typical, but not necessarily “normal” human, it must be obtained on a daily basis through diet and supplementation.
Until most health care practitioners understand that vitamin C is a macronutrient with vitamin qualities and not just a vitamin micronutrient, the importance of restoring the ability of the human liver to synthesize and directly release vitamin C into the blood around the clock will remain unappreciated. After all, who cares if your body is making large amounts of something that you only need in tiny amounts?
Getting the blood levels of vitamin C to remain normal on a 24/7 basis has been shown to be an essential goal in order to maintain and/or restore optimal health. Many studies have clearly established that the higher the blood levels of vitamin C, the longer the life. Much of the literature addresses this in the context of reducing what is known as all-cause mortality. That is to say, sustaining high blood levels of vitamin C decreases the chances of dying from any disease, or cause. This is reflected consistently by studies that assess blood levels of vitamin C, as well as by studies that simply look at the health impact of higher dietary and supplemental intake of vitamin C.
But once the primary “hurdle” of appreciating the enormous role of vitamin C in supporting the optimal function of every cell in the body is understood, it becomes apparent that restoring its endogenous synthesis in the liver “24/7” could well be considered the single most important clinical advancement in the history of medicine, at least to date. This advancement might finally now be available for many, and perhaps most, individuals. As Irwin Stone wrote about vitamin C in his ground-breaking book, The Healing Factor: “…the lack of this molecule in humans has contributed to more deaths, sickness, and just plain misery than any other factor in man’s long history.” He referred to this genetic shortcoming of most people as hypoascorbemia.
Mammalian Vitamin C Synthesis
In nearly all mammals, with the notable exceptions of guinea pigs and some bats, the liver is equipped with a sequence of four enzymes that progressively convert glucose into vitamin C, thereafter releasing it directly into the blood. The formation of each enzyme starts with the precise transcription of a nucleotide sequence in the DNA into a segment of messenger RNA (mRNA). A structure known as a ribosome then attaches itself to this mRNA segment. Upon attachment, the ribosome proceeds linearly down the mRNA segment with the translation of this genetic information, somewhat like a train going down an extent of track. The fourth (last) enzyme in this sequence is L-gulonolactone oxidase (GULO). The actual metabolic pathway is as follows:
D-glucose–>D-glucuronic acid–>L-gulonic acid–>L-gulonolactone–>L-ascorbic acid
It has long been felt that the human liver has an extensively mutated gene, or nucleotide sequence, in the DNA coding for the GULO enzyme, resulting in a genetic defect that is not only incapable of being overcome, but also shared by all humans. In fact, the human GULO gene has been documented to have accumulated a large number of mutations. However, both the guinea pig and human vitamin C GULO genes have also been found to have significant compatibility with the gene of the vitamin C-producing rat. This at least suggests that the guinea pig and human nucleotide sequence shortcomings might be partially overcome in the ribosome-reading translation part of the GULO-producing process.
The amounts of vitamin C per body weight vary widely among vitamin C-producing animals, with cats and dogs producing vitamin C at roughly 10% the degree that a goat can produce. This could indicate that there is an incomplete production (quantity) of a normal vitamin C-producing GULO gene, or it could indicate that there is a larger production of that gene but with multiple incorrect amino acids and only partial functionality (quality). Factors that can impact how well the human vitamin C gene is expressed will be addressed below.
The normal ability of the mammalian liver to synthesize vitamin C from glucose is very dynamic, dramatically increasing in amount when significant new toxin and/or infection-related oxidative stress is encountered in the blood. The physiology in all diseased cells and tissues is ultimately due to the degree and extent to which new biomolecules are oxidized (depleted of electrons). This means that the triggered synthesis of large amounts of electron-donating vitamin C to repair (reduce) oxidized biomolecules while neutralizing new toxins and resolving ongoing oxidation-producing infections is the perfect natural response to promptly bring the organism back to a healthy status. When the redox (reduction-oxidation) status of the body is completely normal, there can be no disease. Of note, the acute presence of oxidative stress in the blood simultaneously triggers a large release of cortisol from the liver as well, the significance of which will be addressed below.
Among mammals who can synthesize vitamin C in their livers, goats in particular have been found to produce some of the largest amounts of vitamin C relative to body weight. In the absence of significant stress (new blood-borne toxins), a 150-pound goat can produce 13,000 mg or more of vitamin C daily. Nevertheless, the amount that the goat routinely produces by itself strongly supports the concept that what is commonly referred to a “megadosing” of vitamin C is woefully less that what should be naturally produced in the optimally-functioning liver. A great deal of the literature will routinely refer to the dosing of a few grams of vitamin C intravenously, or even orally, as a megadose. Such a dosing of vitamin C still falls far short of what the body needs to thrive, especially when faced with an acute oxidative challenge. Giving a sick patient 25 to 100 grams of vitamin C intravenously for an advanced infection, a life-threatening toxin exposure, or an advanced medical condition such as metastatic cancer is much more physiological in amount rather than excessive or extraordinary. Such physiological dosing vastly exceeds what the literature continues to mislabel as a mega-dose, even though that mega-dose vastly exceeds the 50 mg to 250 mg doses utilized in many “failed” clinical studies on the clinical benefits of vitamin C. Although largely ignored, the clinical success of this physiological dosing has been repeatedly established in the medical literature.
The success (and safety) of this level of dosing is further supported by the evidence documenting that significant new toxic or infectious oxidative stresses can make baseline vitamin C production rapidly increase to a striking degree in animals depending on how much new oxidation is in need of reduction. Different acute oxidative stresses have been shown to markedly increase the synthesis of vitamin C in the rat. And, of course, this newly synthesized vitamin C goes directly into the blood, which would require a much higher oral vitamin C dosing to achieve the same result in the human incapable of new vitamin C production. This means that the regular multigram oral dosing of vitamin C is neither extraordinary nor inappropriate in the human making no vitamin C. Instead, such multigram dosing in the human will still be expected to fall short of optimal physiological dosing achieved with normal vitamin C synthesis in the liver.
While an acute and large toxic challenge will result in a large increase in vitamin C production in the vitamin C-producing animal, the effects of smaller but chronic toxin exposures can instead impair such production by oxidizing the critical molecule(s) involved in the production of GULO. However, this will only occur with certain toxins that are chemically suited for attacking the vitamin C-producing apparatus, resulting in epigenetic translation modifications that decrease or stop the production of GULO. But when the toxin does not have such a biochemical specificity, the response of the animal liver will be just to increase vitamin C synthesis to counteract the toxin exposure.
Genetic and Epigenetic Interplay
Many consider genetic diseases or deficiencies to be due to irreparable and relatively large defects in the proper sequence of nucleotides in an extended segment of DNA coding for one or more proteins. When such large defects are present, it is then reasonably concluded that generating sequences of mRNA that are still capable of being translated into biologically active proteins is no longer possible. This is certainly the prevailing attitude toward the inability of the human liver to produce its own vitamin C. However, this does not appear to be correct.
Rather, it appears that the ability of the human liver to fully and accurately translate the mRNA produced by the gene coding for vitamin C is impaired and often completely blocked in most people. Such an inability to properly translate mRNA is one type of epigenetic defect. Oftentimes, such a defect cannot be overcome. Sometimes, however, this defect in translation can be secondary to modifiable factors that otherwise block or impair the ability of the ribosome to accurately translate that mRNA. Additionally, epigenetic defects can also involve certain molecules abnormally binding to the DNA, which can impact mRNA generation and/or integrity and its ultimate translation into a functional target protein.
In a substantial number of genetic diseases, changing only a single base (adenine, cytosine, guanine, or thymine [A, C, G, or T]) in the nucleotide sequence of a single gene can cause a genetic disease by altering the makeup of the codon (sequence of three nucleotides) that corresponds to a specific amino acid. For example, sickle cell anemia results from a single point mutation in the beta hemoglobin gene, resulting in the GAG codon being converted to a GTG codon, which directs the insertion of the amino acid, valine, in place of the proper amino acid, glutamic acid. Other genetic diseases that result from point mutations include color blindness, beta thalassemia, hemophilia, and Duchenne muscular dystrophy.
Of note, there are 64 different 3-base codon sequences in the DNA that can result from the four bases, and three of these serve to encode stop codons in order to end the translation of a segment of messenger RNA. These codons are always present to normally terminate the translation of mRNA by a ribosome, but these codons can also be abnormally present prematurely on the mRNA segment before a complete protein can be translated. Encountering a stop codon early in translation is one prominent example of an epigenetic factor stopping the production of a target protein. However, a genetic disease with the transcription of an incorrect DNA sequence often works in concert with an epigenetic translation defect to prevent the proper formation of the target protein. In that sense, many inherited diseases have both genetic and epigenetic defects. However, generally speaking, the genetic defects (DNA nucleotide sequence) are fixed and the epigenetic defects are sometimes subject to biochemical modifications that can still result in the production of target proteins that have at least partial physiological function.
In the literature, a point mutation, known also as nonsense mutation, produces a single abnormal codon in the messenger RNA needing to be translated into the target protein. When this abnormal codon is one of the three stop codons, the translation of the mRNA is prematurely terminated. This results in an incomplete protein with either reduced or absent function. Sometimes the point mutation just encodes for the wrong amino acid and not a stop codon. This results in a full-length protein, but with the presence of an abnormal amino acid. Such a protein can have minimal to severe dysfunction physiologically, strictly depending on the placement and significance of that particular amino acid in the final protein. Point mutations have been found to account for at least 11% of all genetic defects in humans resulting in inherited disease. Other authors consider such mutations to account for as much as 30% of genetic disorders. Much larger defects in the genomes account for the rest of such diseases, greatly reducing the chances of finding treatment protocols that result in clinically significant improvements since any protein or peptide segments that result from the translation of the mRNA transcribed from such advanced DNA defects would not be expected to function at all.
It has also been documented that when a stop codon is prematurely placed on a segment of mRNA being translated, certain chemicals can result in a “skipping over” of such a stop codon. Such chemicals include the aminoglycoside antibiotics, which were documented to restore normal protein translation in a mouse model of muscular dystrophy. This was considered to be proof of concept information that the ribosome could continue translation through premature stop codons upon administration of the right agent. This “skipping over” phenomenon is known as readthrough, resulting in target protein production in spite of the premature presence of the stop codon. The ability of such abnormally-placed stop codons to be passed over has resulted in a search for other agents that have this ability, preferably without the documented toxicity of the aminoglycosides, especially when taken for a prolonged period or even indefinitel. The target protein can sometimes end up having normal to near-normal function in spite of the earlier presence of the premature stop codon if the final protein is not missing a critically-placed amino acid.
Nearly one-third of the defective genes causing genetic diseases encode for stop codons that prematurely terminate translation into a complete target protein. However, many of these defective genes that encode for stop proteins are extensively mutated and involve far more than just a single point mutation. Such DNA sequences can produce mRNA with codons that encode for multiple abnormal amino acids without the presence of premature-placed stop codons. This can result in full-length target proteins, but containing multiple abnormal amino acids and having variable degrees of physiological activity. Other target proteins can be produced when a premature stop codon is in the mRNA sequence, and an agent that permits readthrough allows a full-length protein with only minimal abnormality in the amino acid sequence. Just like a train proceeding down a track, such agents permit the train (ribosome) to reach its destination after a track defect (stop codon) is repaired or bypassed.
The final protein can be almost completely normal in amino acid sequence, or it can be substantially adulterated with multiple abnormal amino acids in the target protein. This can result in a wide range of protein functionality, from effectively normal to completely nonfunctional, with many intermediate degrees of function. While the ultimate combination of genetic and epigenetic shortcomings in the loss of the ability in the human to make vitamin C remains to be clearly worked out, it nevertheless appears that GULO with sufficient integrity to convert L-gulonolactone into L-ascorbic acid can be achieved when the right epigenetic-modifying agents are ingested. This means that some individuals can regain near-normal GULO synthesis and function, with lesser degrees of synthesis and function regained by others.
Multiple natural and nontoxic molecules have been discovered which appear to readily facilitate the premature stop codon readthrough phenomenon noted above. In cell, animal, and human studies, resveratrol, a nutrient polyphenol found in grapes and wine, was found to induce the production of hemoglobin in beta-thalassemia, a point mutation genetic disease. In 50% of thalassemia patients in one study, resveratrol administration completely eliminated the need for repeated transfusions, appearing to indicate the genetic defect in hemoglobin synthesis had been overcome for those patients, by whatever mechanism.
Vitamin C Synthesis: Initially Present and Gradually Lost
Substantial evidence exists that indicates the ability of the human liver to make vitamin C is present at birth and for a variable amount of time thereafter. It would appear that some epigenetic factors are commonly acquired after birth which work to prevent an accurate and/or complete translation of the mRNA coding for vitamin C. In an early study, the authors asserted that “the amount of ascorbic acid in blood plasma from the umbilical cord blood of infants is from two to four times greater than that in maternal plasma taken at the time of delivery”. In another study, the growing fetus appeared to make a large amount of vitamin C, with brain levels ranging between 400% and 1,100% higher than most adults. Furthermore, the umbilical blood was found to have 400% more vitamin C than the maternal blood. Research on breast-fed babies showed that vitamin C blood levels persisted at a level 200% higher than that of the mother, and that there was no correlation with the vitamin C levels measured in the breast milk. The vitamin C blood levels in the breast-fed infants were also noted to maintain themselves at the same or higher concentrations than the vitamin C-supplemented infants fed by bottle.
In Bantu community infants in South Africa, researchers demonstrated that despite extraordinarily minimal amounts of vitamin C intake (3 to 8 mg/day), the symptoms of scurvy or vitamin C deficiency were never seen. The investigators actually concluded that “the only alternative is to postulate an endogenous production of the vitamin”. It is tempting to speculate that these babies in their relatively isolated “primitive” cultures were exposed to much lower amounts of environmental toxins (food, water, air) than their young peers living in the large cities. Just as with any other medical condition, it is the presence of pro-oxidant toxins that precipitates and maintains disease, and epigenetic defects appear to arise for the same reasons. In a study on mice, it has been shown that their ability to synthesize vitamin C declines markedly over time, likely indicating that the ability to make optimal amounts of vitamin C is not absolute, but directly related to an increased expression of epigenetic defect(s) due to the chronically increased toxin exposures seen with increased age. This reasoning also fits with the concept that decreased vitamin C blood levels play a prominent role in promoting the diseases associated with aging.
Some adults also appear to make their own vitamin C, or at least maintain normal vitamin C blood levels through some other undefined mechanism, whatever that could possibly be. In one study, an adult female continued to show high blood levels of vitamin C as the vitamin C intake was progressively decreased. Another woman went 149 days without any significant dietary vitamin C intake, and she never developed any deficiency symptoms. Similar findings were seen in some other humans and guinea pigs as well.
At the very least, then, the preceding studies are clear that the inability of humans and guinea pigs to maintain normal vitamin C levels is not absolute. The precise mechanism by which some humans can keep vitamin C levels in the normal range without supplementation or very high dietary intake might remain open to speculation for some. However, vitamin C is simply not stored in sizeable amounts anywhere in the body, and the continued presence of normal vitamin C blood levels over an extended period of time in the absence of significant intake can only come from an ongoing internal source of vitamin C, since mobilization from various tissues in the body can simply never come close to supplying the amounts of vitamin C in question into the bloodstream. Internal vitamin C synthesis would appear to be the only logical conclusion.
Restoring Vitamin C Synthesis
Polyphenols are common plant metabolites found in many different fruits and vegetables, as well as coffee and tea. Over 8,000 different polyphenols have been identified, providing a defense against plant pathogens, largely because of their antioxidant properties. Their presence in the human diet also confers antipathogen effects, along with protective effects against heart disease, diabetes, and cancer, as well as many other medical conditions. These polyphenols are also considered to be key agents in modulating the epigenetic factors that promote age-related deterioration and cancer.
In a landmark article, it was shown that the daily supplementation with 45 mg of the polyphenol hydroxytyrosol (HT) markedly increased vitamin C blood levels in 14 healthy volunteers. The primary goal of the study was to establish the safety of HT supplementation, and a wide range of blood parameters were examined, including vitamin C blood levels. While the degree of vitamin C response in all of the study subjects was not identical, the average vitamin C blood level fully doubled at both the 4-week and 8-week point of daily HT administration relative to initial baseline measurements. Furthermore, no negative impacts were seen in a wide array of other biochemical, hematological, vitamin, or mineral parameters that were also measured. The increased vitamin C levels singularly stood out.
HT is found in significant amounts in both olive mill waste and virgin olive oil, and it is also a potent antioxidant by itself in addition to the effects it has on vitamin C blood levels. Multiple studies have documented its positive effects along with other olive oil polyphenols on blood pressure, blood lipids, platelet aggregation, blood clotting, and inflammation in general. A rabbit study looking at supplementation with HT alone documented an improvement in blood lipids, along with an improved antioxidant status and a reduction in the size of atherosclerotic lesions. In vitro cell studies have also shown that HT can work as an iron chelator, decreasing its toxic impact.
In a small unpublished study, a supplement with olive leaf extract containing 50 mg of HT administered daily increased vitamin C blood levels between 50% and 200% in five volunteers after taking it for only a week. Furthermore, in four of the five subjects a clear-cut elevation in those blood levels was seen the day after the first dose. And while the blood levels were not followed over an extensive period of time, significant increases in vitamin C blood levels were still seen 10 days after the stopping of the HT supplement, which had been given daily for a 2-week period.

Regardless of the underlying mechanism, the ability of a properly-dosed HT supplement to elevate blood vitamin C levels in only a day in four of five subjects certainly raises exciting clinical possibilities. It certainly suggests that the treatment of acute infectious and toxic conditions could be given a significant therapeutic boost for most individuals who promptly consume high doses of a quality olive leaf extract upon getting ill. As many patients simply do not want to be bothered with the inconvenience and expense of taking a supplement regularly on a long-term basis, the ability to quickly give a prompt assist to internal vitamin C levels appears to be another highly positive feature of such supplementation.
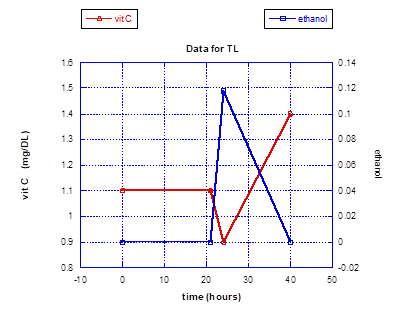
In a small experiment looking at the impact of a large toxin exposure on vitamin C levels in the authors who had been ingesting daily supplementation containing 50 mg of HT for an extended period of time, a striking outcome was realized. The toxin chosen was alcohol to the point of inebriation, with documentation of the peak alcohol blood levels achieved. The first vitamin C levels obtained upon the documentation of high blood alcohol showed a clear and prompt depression. However, the vitamin C levels then dramatically rose and stayed significantly higher for at least 18 hours after the alcohol ingestion. Symptomatically, both subjects felt exceptionally well the following morning, and there was a complete absence of malaise or any other symptoms associated with a hangover. Prior to this small experiment a less quantitative experiment was conducted on just one subject (TL), showing an equally dramatic rise in vitamin C blood levels following the stimulus of a large alcohol intake, except that the elevated blood levels were approximated by vitamin C urine dipstick testing. And as with the blood testing experiment, the urine dipstick indicated a persistent and striking vitamin C elevation for roughly 24 hours.
When a large infectious/toxin presence floods the circulation, an acute depression of vitamin C levels will always occur, as whatever circulating vitamin C is present will be rapidly consumed/oxidized. In the genetically deficient human liver, no new synthesis of vitamin C will ensue and the vitamin C levels will proceed to stay low or even become undetectable. However, upon detecting the presence of this acute oxidative presence in the blood not being quickly extinguished by the existing levels of vitamin C, the normally-functioning liver promptly starts making vitamin C in sufficient amounts to neutralize this oxidative presence in a compensatory manner. And the initial response has to be a dramatic drop in vitamin C levels in order to trigger the new vitamin C synthesis, since the liver can only respond and not anticipate. Furthermore, it appears that the liver continues to make increased amounts of vitamin C until the acute oxidative insult is resolved. It also appears that the resolution of that insult typically occurs with a natural “overshoot” of vitamin C production, resulting often in a sense of unusually good well-being rather than just a return to baseline of clinical “normalcy” after the acute toxin load has been neutralized.
While these small experiments are far from definitive due to size and limitations in the frequency of blood and/or urine testing, the results that were obtained strongly support the initial earlier published study on the impact of HT supplementation on vitamin C blood levels. Also, they clearly support the idea that not only can HT result in more vitamin C in the blood on a chronic basis, but that it can also result in massively larger amounts of vitamin C in the blood when a new large oxidative insult enters the blood. This makes the synthesis of new vitamin C in the liver a very likely reason for these results.
Further testing of the HT would certainly be appropriate and desirable in order to better establish how effective this polyphenol is in restoring vitamin C synthesis, along with establishing how many “nonresponders” exist. The limited data currently indicates that over 90% of supplementers have a significant increase in vitamin C blood levels following HT supplementation. But much detail remains to be worked out, including how sustained such a positive response would be over time. A very simple, proof of concept test would be to simply to look at pre- and post-supplementation GULO blood levels. This would also help quantify the degree of impact, with some subjects going from zero GULO levels in the blood to variable levels of GULO after supplementation. But the appearance of any GULO in the blood after being documented to be absent would be compelling evidence of vitamin C synthesis in the liver getting turned on.
Fight or Flight
The fight or flight response in vitamin C-producing animals is designed to quickly counteract the physiological impact of overwhelming stress, which includes the oxidative stress seen with large toxin exposures and significant infections. In fact, severe enough psychological stress quickly converts to oxidative stress that must be quenched to protect the animal in the same fashion as it is protected from a direct toxic or infectious insult. Practically speaking, both psychological stress and direct toxin/infectious stress serve to provoke a release of cortisol (and adrenaline) from the adrenals at the same time newly-synthesized vitamin C is being released from the liver. This natural coordination between the adrenals and liver of the vitamin C-producing animal rapidly works to increase the vitamin C (antioxidant) presence in the blood and, even more importantly, inside the cells of the body. This surge of new vitamin C, especially with the cortisol effectively pushing it inside the cells, gives the animal the most optimal physiological response to keep the cells from succumbing to the pro-oxidant insult encountered in the blood.
Addressed in greater detail in an earlier OMNS article, vitamin C and cortisol are the two most important anti-inflammatory agents, resolving oxidative stress even better than any prescription agents. The fight or flight response to increased oxidative stress clearly demonstrates that vitamin C and cortisol are actually designed by nature to interact together to optimize the antioxidant impact needed to resolve the disease-causing oxidation that always results from toxins, infections, and stress. Cortisol works to effectively push vitamin C into the cells and rapidly optimize the antioxidant status of those cells. Because of this ability of cortisol to increase vitamin C uptake in the cells, the positive clinical impact of having vitamin C promptly appear in the blood at the same time cortisol is released into the blood cannot be overemphasized. When intracellular vitamin C levels are normalized in cells exposed to a large oxidative insult, the previously increased intracellular oxidative stress is returned to normal levels while any ongoing acute oxidative insult in the blood is neutralized and rendered nontoxic.
Furthermore, the natural design of vitamin C and cortisol working together so effectively underlines the importance of making sure a patient is producing normal amounts of cortisol at rest and upon facing oxidative stress. As discussed in another article, most adults today are not only dealing with acute infections and toxin exposures without the production of new vitamin C in the liver, they also are dealing with the release of suboptimal amounts of cortisol from adrenal glands that are minimally to severely deficient in function.
Finding a physician who is willing to prescribe cortisol (hydrocortisone) to allow you to augment the impact of your daily vitamin C supplementation (or renewed synthesis?) is essential to optimize vitamin C intracellular delivery unless you have perfect cortisol-releasing adrenal function already, which would be very unusual in the older population. To have the optimal chances of restoring some vitamin C synthesis in your body, a quality olive leaf extract providing at least 50 mg of HT daily should be taken for life. However, if lifelong supplementation is not feasible, this supplement should still be taken in higher doses acutely when threatened with a toxic or infectious challenge, since vitamin C levels can increase very quickly in the face of such supplementation. And if your liver does not appear to respond with increased vitamin C synthesis when taking enough olive leaf extract, it is nevertheless an exceptionally good and nontoxic supplement that strongly supports the overall antioxidant status in the body.
Recap
The full importance of vitamin C remains unappreciated by most health care practitioners today, as it is the most important nutrient in the body, and daily intake must be multigram in amount to even approach the benefits that vitamin C affords the body when optimally present. It has been well-established that the higher the blood levels of vitamin C, the longer and healthier the life.
The inability of most human livers to make vitamin C from glucose appears to be a combination of genetic and epigenetic defects. However, it has been discovered that the intake of hydroxytyrosol (HT) in the form of a quality olive leaf extract allows most of the consumers to substantially increase their blood levels of vitamin C. It would appear that HT effectively overcomes an epigenetic translation defect allowing the formation of GULO which can then complete the synthesis of vitamin C in the liver. And while the underlying genetic details remain to be clarified and completely understood, multiple studies have indicated that many humans do make vitamin C in utero and after birth, clearly indicating that the ability to synthesize vitamin C is a lost ability, rather than one that was never present. This also indicates that epigenetic (acquired) defects likely play the major role in adults not having the ability to make vitamin C.
Limited and small experiments have also indicated that humans supplementing HT not only have the return of the ability to make vitamin C, but also the ability to make much larger amounts of vitamin C when faced with acute toxic and/or infectious oxidative stress in the blood. This ability would be profoundly synergistic with all other beneficial treatments for different medical conditions.
Finally, it appears that the human body should not only be making vitamin C, but it should be releasing it at the same time the adrenal glands release cortisol when faced with a substantial new oxidative insult in the blood. Proper supplementation of low-dose cortisol along with vitamin C supplementation can optimize this natural anti-inflammatory synergy.
References:
- Kromhout D, Bloemberg B, Feskens E et al. (2000) Saturated fat, vitamin C and smoking predict long-term population all-cause mortality rates in the Seven Countries Study. International Journal of Epidemiology 29:260-265. PMID: 10817122
- Khaw K, Bingham S, Welch A et al. (2001) Relation between plasma ascorbic acid and mortality in men and women in EPIC-Norfolk prospective study: a prospective population study. European Prospective Investigation into Cancer and Nutrition. Lancet 357:657-663. PMID: 11247548
- Simon J, Hudes E, Tice J (2001) Relation of serum ascorbic acid to mortality among US adults. Journal of the American College of Nutrition 20:255-263. PMID: 11444422
- Khaw K, Wareham N, Bingham S et al. (2008) Combined impact of health behaviors and mortality in men and women: the EPIC-Norfolk prospective population study. PLoS Medicine 5:e12. PMID: 18184033
- Ford E, Li C, Cunningham T, Croft J (2014) Associations between antioxidants and all-cause mortality among US adults with obstructive lung disease. The British Journal of Nutrition 112:1662-1673. PMID: 25315508
- Sotomayor C, Eisenga M, Neto A et al. (2017) Vitamin C depletion and all-cause mortality in renal transplant recipients. Nutrients 9:568. PMID: 28574431
- Aune D, Keum N, Giovannucci E et al. (2018) Dietary intake and blood concentrations of antioxidants and the risk of cardiovascular disease, total cancer, and all-cause mortality: a systematic review and dose-response meta-analysis of prospective studies. The American Journal of Clinical Nutrition 108:1069-1091. PMID: 30475962
- Jayedi A, Rashidy-Pour A, Parohan M et al. (2018) Dietary antioxidants, circulating antioxidant concentrations, total antioxidant capacity, and risk of all-cause mortality: a systematic review and dose-response meta-analysis of prospective observational studies. Advances in Nutrition 9:701-716. PMID: 30239557
- Wang S, Fan J, Taylor P et al. (2018) Association of plasma vitamin C concentration to total and cause-specific mortality: a 16-year prospective study in China. Journal of Epidemiology and Community Health 72: 1076-1082. PMID: 30100578
- Sheng L, Jiang Y, Pan A, Koh W (2022) Dietary total antioxidant capacity and mortality outcomes: the Singapore Chinese Health Study. European Journal of Nutrition Feb 5. Online ahead of print. PMID: 35122488
- Xu K, Peng R, Zou Y et al. (2022) Vitamin C intake and multiple health outcomes: an umbrella review of systematic reviews and meta-analyses. International Journal of Food Sciences and Nutrition Mar 15. Online ahead of print. PMID: 35291895
- Stone I (1972) The Healing Factor: “Vitamin C” Against Disease. New York, NY: Grosset & Dunlap
- Stone I (1967) The genetic disease, hypoascorbemia. A fresh approach to an ancient disease and some of its medical implications. Acta Geneticae Medicae et Gemellologiae 16:52-62. PMID: 6063937
- Chatterjee I (1973) Evolution and the biosynthesis of ascorbic acid. Science 182:1271-1272. PMID: 4752221
- Chatterjee I (2009) The history of vitamin C research in India. Journal of Biosciences 34:185-194. PMID: 19550033
- Nishikimi M, Yagi K (1991) Molecular basis for the deficiency in humans of gulonolactone oxidase, a key enzyme for ascorbic acid biosynthesis. The American Journal of Clinical Nutrition 54:1203S-1208S. PMID: 1962571
- Nishikimi M, Koshisaka T, Ozawa T, Yagi K (1988) Occurrence in humans and guinea pigs of the gene related to their missing enzyme L-gulono-gamma-lactone oxidase. Archives of Biochemistry and Biophysics 267:842-846. PMID: 3214183
- Chatterjee I, Majumder A, Nandi B, Subramanian N (1975) Synthesis and some major functions of vitamin C in animals. Annals of the New York Academy of Sciences 258:24-47. PMID: 1106297
- Klenner F (1951) Massive doses of vitamin C and the virus diseases. Southern Medicine and Surgery 113:101-107. PMID: 14855098
- Klenner F (1953) The use of vitamin C as an antibiotic. Journal of Applied Nutrition 6:274-278.
- Klenner F (1971) Observations on the dose and administration of ascorbic acid when employed beyond the range of a vitamin in human pathology. Journal of Applied Nutrition Winter, pp. 61-88.
- Klenner F (1974) Significance of high daily intake of ascorbic acid in preventive medicine. Journal of Preventive Medicine 1:45-69.
- Cathcart R (1981) Vitamin C, titrating to bowel tolerance, anascorbemia, and acute induced scurvy. Medical Hypotheses 7:1359-1376. PMID: 7321921
- Cathcart R. (1984) Vitamin C in the treatment of acquired immune deficiency syndrome (AIDS). Medical Hypotheses 14:423-433. PMID: 6238227
- Cathcart R (1985) Vitamin C: the nontoxic, nonrate-limited, antioxidant free radical scavenger. Medical Hypotheses 18:61-77. PMID: 4069036
- Landwehr R (1991) The origin of the 42-year stonewall of vitamin C. Journal of Orthomolecular Medicine 6:99-103. http://www.orthomolecular.org/library/jom/1991/pdf/1991-v06n02-p099.pdf
- Jackson J, Riordan H, Bramhall N, Neathery S (2002) Sixteen-year history with high dose intravenous vitamin C treatment for various types of cancer and other diseases. Journal of Orthomolecular Medicine 17:117-119. http://orthomolecular.org/library/jom/2002/pdf/2002-v17n02-p117.pdf
- Riordan H, Casciari J, Gonzalez M et al. (2005) A pilot clinical study of continuous intravenous ascorbate in terminal cancer patients. Puerto Rico Health Sciences Journal 24:269-276. PMID: 16570523
- Padayatty S, Riordan H, Hewitt S et al. (2006) Intravenously administered vitamin C as cancer therapy: three cases. CMAJ 174:937-942. PMID: 16567755
- Mikirova N, Jackson J, Riordan N (2007) The effect of high dose IV vitamin C on plasma antioxidant capacity and level of oxidative stress in cancer patients and healthy subjects. Journal of Orthomolecular Medicine 22:153-160. http://orthomolecular.org/library/jom/2007/pdf/2007-v22n03-p153.pdf
- Marcial-Vega V, Gonzalez-Terron G, Levy T (2015) Intravenous ascorbic acid and hydrogen peroxide in the management of patients with Chikungunya. Boletin de la Asociacion Medica de Puerto Rico 107:20-24. PMID: 26035980
- Subramanian N, Nandi B, Majumder A, Chatterjee I (1973) Role of L-ascorbic acid on detoxification of histamine. Biochemical Pharmacology 22:1671-1673. PMID: 4147115
- Stone I (1979) Homo sapiens ascorbicus, a biochemically corrected robust human mutant. Medical Hypotheses 5:711-721. PMID: 491997
- Conney A, Bray G, Evans C, Burns J (1961) Metabolic interactions between L-ascorbic acid and drugs. Annals of the New York Academy of Sciences 92:115-127. PMID: 13695066
- Touster O, Hollmann S (1961) Nutritional and enzymatic studies on the mechanism of stimulation of ascorbic acid synthesis by drugs and carcinogenic hydrocarbons. Annals of the New York Academy of Sciences 92:318-323. PMID: 13777750
- Aarts E (1966) Differentiation of the barbiturate stimulation of the glucuronic acid pathway from de novo enzyme synthesis. Biochemical Pharmacology 15:1469-1477. PMID: 4382013
- Chatterjee I, Chatterjee G, Ghosh N et al. (1960) Biological synthesis of L-ascorbic acid in animal tissues: conversion of L-gulonolactone into L-ascorbic acid. The Biochemical Journal 74:193-203. PMID: 13809446
- Srinivasan T (2011) Genetics, epigenetics, and pregenetics. International Journal of Yoga 4:47-48. PMID: 22022121
- Kubota T, Miyake K, Hirasawa T (2012) Epigenetic understanding of gene-environment interactions in psychiatric disorders: a new concept of clinical genetics. Clinical Epigenetics 4:1. PMID: 22414323
- Moosavi A, Ardekani A (2016) Role of epigenetics in biology and human diseases. Iranian Biomedical Journal 20:246-258. PMID: 27377127
- Isac T, Isac S, Rababoc R et al. (2022) Epigenetics in inflammatory liver diseases: a clinical perspective (Review). Experimental and Therapeutic Medicine 23:366. PMID: 35481220
- Zima L, West R, Smolen P et al. (2022) Epigenetic modifications and their potential contributions to traumatic brain injury pathobiology and outcome. Journal of Neurotrauma Apr 28. Online ahead of print. PMID: 35481812
- Pace B, Starlard-Davenport A, Kutlar A (2021) Sickle cell disease: progress towards combination drug therapy. British Journal of Haematology 194:240-251. PMID: 33471938
- Palma M, Lejeune F (2020) Deciphering the molecular mechanism of stop codon readthrough. Biological Reviews of the Cambridge Philosophical Society 96:310-329. PMID: 33089614
- Wangen J, Green R (2020) Stop codon context influences genome-wide stimulation of termination codon readthrough by aminoglycosides. eLife 9:e52611. PMID: 31971508
- Mort M, Ivanov D, Cooper D, Chuzhanova N (2008) A meta-analysis of nonsense mutations causing human genetic disease. Human Mutation 29:1037-1047. PMID: 18454449
- Martins-Dias P, Romao L (2021) Nonsense suppression therapies in human genetic diseases. Cellular and Molecular Life Sciences 78:4677-4701. PMID: 33751142
- Miller J, Pearce D (2014) Nonsense-medicated decay in genetic disease: friend or foe? Mutation Research. Reviews in Mutation Research 762:52-64. PMID: 25485595
- Bidou L, Allamand V, Rousset J, Namy O (2012) Sense from nonsense: therapies for premature stop codons. Trends in Molecular Medicine 18:679-688. PMID: 23083810
- Yesmin F, Bhuiyan R, Ohmi Y et al. (2020) Aminoglycosides are efficient reagents to induce readthrough of premature termination codon in mutant B4GALNT1 genes found in families of hereditary spastic paraplegia. Journal of Biochemistry 168:103-112. PMID: 32282910
- Beryozkin A, Samanta A, Gopalakrishnan P et al. (2022) Translational read-through drugs (TRIDs) are able to restore protein expression and ciliogenesis in fibroblasts of patients with retinitis pigmentosa caused by a premature termination codon in FAM161A. International Journal of Molecular Sciences 23:3541. PMID: 35408898
- Keeling K, Bedwell D (2011) Suppression of nonsense mutations as a therapeutic approach to treat genetic diseases. Wiley Interdisciplinary Reviews. RNA 2:837-852. PMID: 21976286
- Lee H, Dougherty J (2012) Pharmaceutical therapies to recode nonsense mutations in inherited diseases. Pharmacology & Therapeutics 136:227-266. PMID: 22820013
- Keeling K, Xue X, Gunn G, Bedwell D (2014) Therapeutics based on stop codon readthrough. Annual Review of Genomics and Human Genetics 15:371-394. PMID: 24773318
- Lombardi S, Testa M, Pinotti M, Branchini A (2020) Molecular insights into determinants of translational readthrough and implications for nonsense suppression approaches. International Journal of Molecular Sciences 21:9449. PMID: 33322589
- Politano L (2021) Read-through approach for stop mutations in Duchenne muscular dystrophy. An update. Acta Myologica 40:43-50. PMID: 33870095
- Linde L, Kerem B (2008) Introducing sense into nonsense in treatments of human genetic diseases. Trends in Genetics 24:552-563. PMID: 18937996
- Bianchi N, Zuccato C, Lampronti I et al. (2009) Fetal hemoglobin inducers from the Natural World: A novel approach for identification of drugs for the treatment of {beta}-thalassemia and sickle-cell anemia. Evidence-Based Complementary and Alternative Medicine 6:141-151. PMID: 18955291
- Sermet-Gaudelus I, Namy O (2016) New pharmacological approaches to treat patients with cystic fibrosis with nonsense mutations. American Journal of Respiratory and Critical Care Medicine 194:1042-1044. PMID: 27797609
- Mutyam V, Du M, Xue X et al. (2016) Discovery of clinically approved agents that promote suppression of cystic fibrosis transmembrane conductance regulator nonsense mutations. American Journal of Respiratory and Critical Care Medicine 194:1092-1103. PMID: 27104944
- Fibach E, Prus E, Bianchi N et al. (2012) Resveratrol: antioxidant activity and induction of fetal hemoglobin in erythroid cells from normal donors and β-thalassemia patients. International Journal of Molecular Medicine 29:974-982. PMID: 22378234
- Franco S, De Falco L, Ghaffari S et al. (2014) Resveratrol accelerates erythroid maturation by activation of FoxO3 and ameliorates anemia in beta-thalassemic mice. Haematologica 99:267-275. PMID: 23975182
- Chowdhury et al. (2017) International Journal of Advanced Research 5:1816-1821.
- Teel H, Burke B, Draper B (1938) Vitamin C in human pregnancy and lactation. American Journal of Diseases of Children 56:1004-1010.
- Adlard B, De Souza S, Moon S (1974) Ascorbic acid in fetal human brain. Archives of Disease in Childhood 49:278-282. PMID: 4830116
- Salmenpera L (1984) Vitamin C nutrition during prolonged lactation: optimal in infants while marginal in some mothers. The American Journal of Clinical Nutrition 40:1050-1056. PMID: 6496385
- Andersson M, Walker A, Falcke H (1956) An investigation of the rarity of infantile scurvy among the South African Bantu. The British Journal of Nutrition 10:101-105. PMID: 13315928
- Iwama M, Amano A, Shimokado K et al. (2012) Ascorbic acid levels in various tissues, plasma and urine of mice during aging. Journal of Nutritional Science and Vitaminology 58:169-174. PMID: 22878386
- Cummings M (1981) Can some people synthesize ascorbic acid? The American Journal of Clinical Nutrition 34:297-298. PMID: 7211730
- Williams R, Deason G (1967) Individuality in vitamin C needs. Proceedings of the National Academy of Sciences of the United States of America 57:1638-1641. PMID: 5231398
- Odumosu A, Wilson C (1971) Metabolic availability of ascorbic acid in female guinea-pigs. British Journal of Pharmacology 42:637P-638P. PMID: 5116040
- Ginter E (1976) Ascorbic acid synthesis in certain guinea pigs. International Journal for Vitamin and Nutrition Research 46:173-179. PMID: 1032629
- Khan H, Sureda A, Belwal T et al. (2019) Polyphenols in the treatment of autoimmune diseases. Autoimmunity Reviews 18:647-657. PMID: 31059841
- Mokni M, Limam F, Elkahoui S et al. (2007) Strong cardioprotective effect of resveratrol, a red wine polyphenol, on isolated rat hearts after ischemia/reperfusion injury. Archives of Biochemistry and Biophysics 457:1-6. PMID: 17125727
- Du G, Zhang Z, Wen X et al. (2012) Epigallocatechin gallate (EGCG) is the most effective cancer chemopreventive polyphenol in green tea. Nutrients 4:1679-1691. PMID: 23201840
- Steinmann J, Buer J, Pietschmann, Steinmann E (2013) Anti-infective properties of epigallocatechin-3-gallate (EGCG), a component of green tea. British Journal of Pharmacology 168:1059-1073. PMID: 23072320
- Grootaert C, Kamiloglu S, Capanoglu E, Camp J (2015) Cell systems to investigate the impact of polyphenols on cardiovascular health. Nutrients 7:9229-9255. PMID: 26569293
- Umeno A, Horie M, Murotomi K et al. (2016) Antioxidative and antidiabetic effects of natural polyphenols and isoflavones. Molecules 21:708. PMID: 27248987
- Chu A (2022) Quarter-century explorations of bioactive polyphenols: diverse health benefits. Frontiers in Bioscience 27:134. PMID: 35468693
- Arora I, Sharma M, Sun L, Tollefsbol T (2020) The epigenetic link between polyphenols, aging and age-related diseases. Genes 11:1094. PMID: 32962067
- Selvakumar P, Badgeley A, Murphy P et al. (2020) Flavonoids and other polyphenols act as epigenetic modifiers in breast cancer. Nutrients 12:761. PMID: 32183060
- Ganguly S, Arora I, Tollefsbol T (2021) Impact of stilbenes as epigenetic modulators of breast cancer risk and associated biomarkers. International Journal of Molecular Sciences 22:10033. PMID: 34576196
- Kansal V, Agarwal A, Harbour A et al. (2022) Regular intake of green tea polyphenols suppresses the development of nonmelanoma skin cancer through miR-29-mediated epigenetic modifications. Journal of Clinical Medicine 11:398. PMID: 35054091
- Lopez-Huertas E, Fonolla J (2017) Hydroxytyrosol supplementation increases vitamin C levels in vivo. A human volunteer trial. Redox Biology 11:384-389. PMID: 28063380
- Tripoli E, Giammanco M, Tabacchi G et al. (2005) The phenolic compounds of olive oil: structure, biological activity and beneficial effects on human health. Nutrition Research Reviews 18:98-112. PMID: 19079898
- Fito M, Gimeno E, Covas M et al. (2002) Postprandial and short-term effects of dietary virgin olive oil on oxidant/antioxidant status. Lipids 37:245-251. PMID: 11942474
- Leger C, Carbonneau M, Michel F et al. (2005) A thromboxane effect of a hydroxytyrosol-rich olive oil wastewater extract in patients with uncomplicated type I diabetes. European Journal of Clinical Nutrition 59:727-730. PMID: 15798774
- Dell’Agli M, Maschi O, Galli G et al. (2008) Inhibition of platelet aggregation by olive oil phenols via cAMP-phosphodiesterase. The British Journal of Nutrition 99:945-951. PMID: 17927845
- Moreno-Luna R, Munoz-Hernandez R, Miranda M et al. (2012) Olive oil polyphenols decrease blood pressure and improve endothelial function in young women with mild hypertension. American Journal of Hypertension 25:1299-1304. PMID: 22914255
- Gonzalez-Santiago M, Martin-Bautista E, Carrero J et al. (2006) One-month administration of hydroxytyrosol, a phenolic antioxidant present in olive oil, to hyperlipemic rabbits improves blood lipid profile, antioxidant status and reduces atherosclerosis development. Atherosclerosis 188:35-42. PMID: 16300770
- Chimi H, Morel I, Lescoat G et al. (1995) Inhibition of iron toxicity in rat hepatocyte culture by natural phenolic compounds. Toxicology In Vitro 9:695-702. PMID: 20650146
- Kitsati N, Mantzaris M, Galaris D (2016) Hydroxytyrosol inhibits hydrogen peroxide-induced apoptotic signaling via labile iron chelation. Redox Biology 10:233-242. PMID: 27810738
- Levy T (2021) Vitamin C and cortisol: synergistic infection and toxin defense. http://orthomolecular.org/resources/omns/v17n28.shtml
- Levy T (2022) How COVID helped me regain good health. http://orthomolecular.org/resources/omns/v18n06.shtml
Nutritional Medicine is Orthomolecular Medicine
Orthomolecular medicine uses safe, effective nutritional therapy to fight illness. For more information: http://www.orthomolecular.org
Find a Doctor
To locate an orthomolecular physician near you: http://orthomolecular.org/resources/omns/v06n09.shtml
The peer-reviewed Orthomolecular Medicine News Service is a non-profit and non-commercial informational resource.
Editorial Review Board:
Albert G. B. Amoa, MB.Ch.B, Ph.D. (Ghana)
Seth Ayettey, M.B., Ch.B., Ph.D. (Ghana)
Ilyès Baghli, M.D. (Algeria)
Ian Brighthope, MBBS, FACNEM (Australia)
Gilbert Henri Crussol, D.M.D. (Spain)
Carolyn Dean, M.D., N.D. (USA)
Ian Dettman, Ph.D. (Australia)
Susan R. Downs, M.D., M.P.H. (USA)
Ron Ehrlich, B.D.S. (Australia)
Hugo Galindo, M.D. (Colombia)
Martin P. Gallagher, M.D., D.C. (USA)
Michael J. Gonzalez, N.M.D., D.Sc., Ph.D. (Puerto Rico)
William B. Grant, Ph.D. (USA)
Claus Hancke, MD, FACAM (Denmark)
Tonya S. Heyman, M.D. (USA)
Patrick Holford, BSc (United Kingdom)
Suzanne Humphries, M.D. (USA)
Ron Hunninghake, M.D. (USA)
Bo H. Jonsson, M.D., Ph.D. (Sweden)
Dwight Kalita, Ph.D. (USA)
Felix I. D. Konotey-Ahulu, MD, FRCP, DTMH (Ghana)
Jeffrey J. Kotulski, D.O. (USA)
Peter H. Lauda, M.D. (Austria)
Alan Lien, Ph.D. (Taiwan)
Homer Lim, M.D. (Philippines)
Stuart Lindsey, Pharm.D. (USA)
Pedro Gonzalez Lombana, MD, MsC, PhD (Colombia)
Victor A. Marcial-Vega, M.D. (Puerto Rico)
Juan Manuel Martinez, M.D. (Colombia)
Mignonne Mary, M.D. (USA)
Jun Matsuyama, M.D., Ph.D. (Japan)
Joseph Mercola, D.O. (USA)
Jorge R. Miranda-Massari, Pharm.D. (Puerto Rico)
Karin Munsterhjelm-Ahumada, M.D. (Finland)
Tahar Naili, M.D. (Algeria)
W. Todd Penberthy, Ph.D. (USA)
Zhiyong Peng, M.D. (China)
Isabella Akyinbah Quakyi, Ph.D. (Ghana)
Selvam Rengasamy, MBBS, FRCOG (Malaysia)
Jeffrey A. Ruterbusch, D.O. (USA)
Gert E. Schuitemaker, Ph.D. (Netherlands)
T.E. Gabriel Stewart, M.B.B.CH. (Ireland)
Thomas L. Taxman, M.D. (USA)
Jagan Nathan Vamanan, M.D. (India)
Garry Vickar, M.D. (USA)
Ken Walker, M.D. (Canada)
Anne Zauderer, D.C. (USA)
Andrew W. Saul, Ph.D. (USA), Editor-In-Chief
Associate Editor: Robert G. Smith, Ph.D. (USA)
Editor, Japanese Edition: Atsuo Yanagisawa, M.D., Ph.D. (Japan)
Editor, Chinese Edition: Richard Cheng, M.D., Ph.D. (USA)
Editor, French Edition: Vladimir Arianoff, M.D. (Belgium)
Editor, Norwegian Edition: Dag Viljen Poleszynski, Ph.D. (Norway)
Editor, Arabic Edition: Moustafa Kamel, R.Ph, P.G.C.M (Egypt)
Editor, Korean Edition: Hyoungjoo Shin, M.D. (South Korea)
Editor, Spanish Edition: Sonia Rita Rial, PhD (Argentina)
Contributing Editor: Thomas E. Levy, M.D., J.D. (USA)
Contributing Editor: Damien Downing, M.B.B.S., M.R.S.B. (United Kingdom)
Assistant Editor: Helen Saul Case, M.S. (USA)
Technology Editor: Michael S. Stewart, B.Sc.C.S. (USA)
Associate Technology Editor: Robert C. Kennedy, M.S. (USA)
Legal Consultant: Jason M. Saul, JD (USA)
Comments and media contact: drsaul@doctoryourself.com OMNS welcomes but is unable to respond to individual reader emails. Reader comments become the property of OMNS and may or may not be used for publication.
Source: https://orthomolecular.activehosted.com
